Personnel who put together, dispense, and administer CSPs ought to store them strictly in accordance While using the conditions stated about the label of component solutions and concluded CSPs. When CSPs are known to have been exposed to temperatures warmer in comparison to the warmest labeled Restrict, but not exceeding forty
Component of aseptic processing in which a pre-sterilized item is filled and/or packaged into sterile or depyrogenated containers and partly closed and/or shut
Media fill test inspection coaching and qualification are actually finished for personnel assigned to media fill test/inspection.
Units could be incubated upright when they are actually inverted to wet al interior surfaces with media. Media may additionally be incubated inverted.
it is a technique by which a liquid microbiological nutrient growth medium is prepared and filled inside of a simulation of regular manufacturing Procedure Study much less
As the Holiday Season approaches, we wish to continue to keep you informed about our upcoming closures to ensure a seamless expertise for both you and your laboratory.
If container / closure defects are detected all through post incubation inspection, the root cause of the defect needs to be investigated having a corrective motion.
All manufacturing procedures in pharmaceutical marketplace has to be validated. This need is stated in the European Pharmacopoeia:eighteen “Approach validation contain checks on the procedure here are routinely carried out by way of method simulation tests using microbial progress media which happen to be then incubated and examined for microbial contamination (media fill tests).“
Other uncategorized cookies are those that are now being analyzed and have not been categorized into a class as nonetheless. Help save & Settle for
9.7.5 If your cause is not assignable, then the procedure need to be validated, as This is a new procedure. Consecutive a few-approach simulation test really should be executed to demonstrate consistency and dependability over the sterile formulation manufacturing procedure to supply satisfactory solution.
Sterile powder fills or simulation of sterile suspensions involves using sterilized powders, including Lactose, that won't inhibit the website growth of organisms and will not interfere with the chance to detect expansion during the inspection.
The media should be handed the test for GPT to promote The expansion of gram-detrimental and gram-optimistic bacteria and yeast and molds.
We appreciate your cooperation and knowledge in the course of this holiday getaway time period. Thanks for selecting Hardy Diagnostics as your reliable associate.
Environmental monitoring internet sites, like staff gowns and gloves, are already selected as well as their Health of use justified. Initial at- rest environmental monitoring qualification has become accomplished for the line/location and summary reports are reviewed and permitted by QA.
 Tony Danza Then & Now!
Tony Danza Then & Now!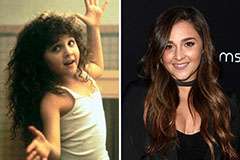 Alisan Porter Then & Now!
Alisan Porter Then & Now! Jennifer Love Hewitt Then & Now!
Jennifer Love Hewitt Then & Now! Kerri Strug Then & Now!
Kerri Strug Then & Now! The Olsen Twins Then & Now!
The Olsen Twins Then & Now!